Introduction
TP53 is a tumor suppressor protein encoded by the TP53 gene that plays a pivotal role in maintaining genomic stability in response to DNA damage, activates DNA repair programs and triggers cell-cycle arrest. Mutations in TP53 (TP53-mut) are present in approximately 5-10% of de novo myelodysplastic syndromes (MDS) and acute myeloid leukemias (AML), and confer an extremely poor prognosis, irrespectively of the treatment administered. The frequency of TP53 abnormalities increases up to 25-40% in therapy-related MDS and AML, up to 70%-80% in patients with complex karyotype and in patients with loss of chromosome 17/17p, 5/5q, or 7/7q. “Multihit” TP53-mut present with the loss of both wildtype TP53-alleles, either through point mutations or larger aberrations to chromosome 17, in complex monosomal karyotypes. Recent data strongly support the concept that TP53mut, particularly multi-hit TP53, results in similarly poor clinical outcomes, regardless of its classification as MDS or AML, arguing for a revised TP53 mutant myeloid entity that includes both MDS and AML. The aim of our analysis is to assess the impact of the multihit TP53-mut on clinical outcomes according to the type of treatment received. We will particularly focus on the presence of mono or biallelic mutation status of TP53 mutation.
Methods
We retrospectively evaluated 42 patients with TP53 mutated MDS or AML diagnosed from October 2016 to June 2023 in our department at the Catalan Institute of Oncology-L'Hospitalet in Spain. We analyze the prognosis of these two diseases, focusing on single-hit or multi-hit status of TP53 mutation and treatments received.
Results
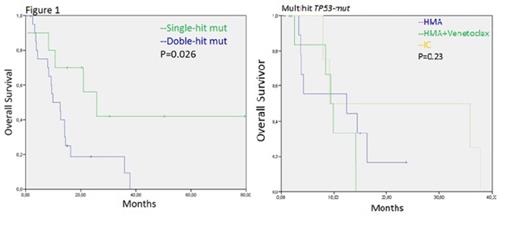
Median age at diagnosis was 71 years (range, 44-87 years) with a male predominance (67%). Thirty-five (84%) patients were diagnosed of AML and 7 (16%) of MDS. Among AML patients, 17% had a prior diagnosis of dysplasia, 78% were diagnosed of AML with dysplasia-related changes, 13% with therapy-related neoplasms, and 9% were AML with maturation and/or AML with mutated NPM1. Regarding MDS patients, 57% had an excess of blast-2 (EB2) MDS, 43% MDS multilineage dysplasia (MLD) and MDS with excess of blast-1(EB1). Complex karyotype was present in 79% of the patients. The median variant allele frequency (VAF) of TP53mut was 36%. Multi-hit TP53 status was observed in 24 patients (57%). In this group of multi-hit TP53mut median age was 72 years (range,44-81 years) with a male predominance (67%), the median VAF of TP53mut was 40%. Twenty (83%) patients had MDS diagnosis and 4 (17%) had AML diagnosis; all of them presented complex karyotype. In the whole group the most concurrent mutations associated with TP53 were TET2 in 17%, ASXL1 in 14% and DNMT3A, NRAS y CREBBP in 12% of patients. Intensive chemotherapy (IC) was administrated as first-line treatment in 17% of patients, 45% received hypomethylating agents (HMA) monotherapy, 24% venetoclax-based combination with HMA, and 14% exclusively supportive treatment. 16 patients (38%) achieved a complete response (CR), 44%, 31% and 25% with HMA+venetoclax, IC and with HMA monotherapy, respectively. Eight patients (19%) achieved a partial response (PR), 88% with HMA and 12% with IC, 7 patients (17%) stable disease, 86% with HMA monotherapy and 14% with HMA+venetoclax and 2 patients (5%) were refractory to IC. In 2 patients (5%), disease was not evaluable. The treatment related mortality was 4.2%. Median overall survival (OS) was 12.5 months [IC 95 % (0.0, 23.54)] and median progression free survival (PFS) was 9, 2 months [IC 95% (3.2, 32.6)]. Median OS of patients with multihit TP53 was 9.8 months, vs 25.7 months in patients with single-hit TP53-mut (P=0.026) (Figure1). Median PFS of the patients with multi-hit and single-hit TP53 was 9.1 and 11.6 months (P=0.07), respectively. The impact of multi-hit TP53 on OS and PFS according to the treatment received (HMA monotherapy vs HMA + venetoclax vs IC) was not statistically significant, just like in the single-hit TP53 (Figure1).
Conclusions
The presence of multihit TP53 -mut is a prognostic variable for survival in our group of patients analyzed with TP53-Mut. Survival is poor, around one year, in this group of patients independently on the treatment strategy received; this fact would call into question the benefit of IC with the toxicity it entails, in this group of patients. Further development of new effective therapies for multihit TP53-mut AML and MDS is needed.
Disclosures
Sureda Balari:Pierre Fabre: Consultancy, Honoraria; Astra Zeneca: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Kite: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Jannsen: Consultancy, Honoraria; MSD: Consultancy, Honoraria; BMS/Celgene: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding, Speakers Bureau; GenMab: Consultancy, Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal